 A low-cost, safe, and effective contraceptive implant
A low-cost, safe, and effective contraceptive implant
Zarin®, Levoplant, also known as Sino-implant (II), is a subcutaneous contraceptive implant manufactured in China by Shanghai Dahua Pharmaceutical Company Limited. The implant is composed of two thin, flexible rods made of medical grade silicone, each containing 75 mg of levonorgestrel (a synthetic progestin). Each rod consists of a drug core and an external medical silastic tube that is sealed at both ends with medical adhesive. The implant is inserted under the skin of the upper arm.
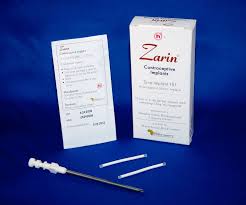 Zarin® has the same mechanism of action as Jadelle. Like other contraceptive implants, Zarin®, is one of the most highly effective contraceptive methods with an annual pregnancy rate below 1%. Clinical longitudinal trials published in peer-reviewed journals with a population of 11,800 women followed for up to five years show Zarin to be both safe, and effective. The implants are currently labeled for four years of use.
Zarin® has the same mechanism of action as Jadelle. Like other contraceptive implants, Zarin®, is one of the most highly effective contraceptive methods with an annual pregnancy rate below 1%. Clinical longitudinal trials published in peer-reviewed journals with a population of 11,800 women followed for up to five years show Zarin to be both safe, and effective. The implants are currently labeled for four years of use.
Zarin® has been available in China since 1994, and has been routinely exported to Indonesia during the past five years. To date, around six million units have been distributed worldwide.
 Shanghai Dahua Pharmaceutical Co. Ltd.
Shanghai Dahua Pharmaceutical Co. Ltd.
Dahua Pharmaceuticals is an enterprise registered in China by the State Food and Drug Administration and designated by the State Population and Family Planning Commission for the manufacture of contraceptive products. The current manufacturing facility opened in 2004 and was specifically designed and built to meet China’s Good Manufacturing Practice (GMP) standards.
- Built for the manufacture of contraceptive products
- GMP Certified by the People’s Republic of China
- ISO 9001 and ISO 13485 certified: National Quality Institute (NQI, UK)
- Six million implants distributed have been distributed worldwide
Ongoing Product Quality Evaluation
Ongoing and recurrent quality evaluation of Zarin® is being performed to ensure the product meets standards. The initial evaluation of several manufacturing lots confirmed that Zarin® met lot release specifications. Each lot was tested, with the Chinese Pharmacopeia testing methods, by both Family Health International’s (FHI) Product Quality and Compliance Laboratories and by an independent contract laboratory.
Lot release testing verification will continue for the next five years. This will be done as a standard practice prior to distribution in countries where Zarin® is registered through this initiative.
Through a series of independent audits conducted by qualified and trained auditors, Dahua Pharmaceutical’s has pledged compliance with Good Manufacturing Practice.
The product is also registered in Kenya, Sierra Leone, Madagascar, Uganda, Zambia, Malawi and Mozambique. Registration in other African countries is currently underway. Shanghai Dahua Pharmaceuticals Limited plant has been successfully audited for certificate of Good Manufacturing Practices by a number of National Regulatory Authorities.
Pharm Access Africa Limited also supports a pharmaco-vigilance hub for Zarin®, in Nairobi, Kenya that monitors adverse events with the product. These are shared with national regulatory authorities, manufacturing site and partners.
Exclusive Distribution Rights and Public Sector Pricing
Zarin® is Pharm Access Africa Limited’s brand name for Sino Implant (ll). FHI360 facilitates the negotiation of six-year contracts between Shanghai Dahua Pharmaceuticals Limited and prospective partners to purchase Sino-implant (II) at the lowest possible price. These contracts in turn will provide partners with exclusive distribution rights.
Through Pharm Access Africa Limited, Zarin® is offered with disposable trocar in a 1:1 ratio.
 A low-cost, safe, and effective contraceptive implant
A low-cost, safe, and effective contraceptive implant
 Zarin® has the same mechanism of action as Jadelle. Like other contraceptive implants, Zarin®, is one of the most highly effective contraceptive methods with an annual pregnancy rate below 1%. Clinical longitudinal trials published in peer-reviewed journals with a population of 11,800 women followed for up to five years show Zarin to be both safe, and effective. The implants are currently labeled for four years of use.
Zarin® has the same mechanism of action as Jadelle. Like other contraceptive implants, Zarin®, is one of the most highly effective contraceptive methods with an annual pregnancy rate below 1%. Clinical longitudinal trials published in peer-reviewed journals with a population of 11,800 women followed for up to five years show Zarin to be both safe, and effective. The implants are currently labeled for four years of use. Shanghai Dahua Pharmaceutical Co. Ltd.
Shanghai Dahua Pharmaceutical Co. Ltd.